Abstract
Introduction
Bruton's tyrosine kinase (BTK) inhibitors have recently been shown to be safe and highly active as monotherapy in Waldenström macroglobulinemia (WM). Immune checkpoint inhibitors are being actively investigated in many lymphoid malignancies, and the expression of programmed cell death protein 1 (PD-1) and its ligands on malignant B cells in WM provides a rationale for the use of PD-1 inhibitors.
Two patients with relapsed WM were treated with a novel anti-PD-1 antibody (BGB-A317) in combination with BGB-3111, a BTK inhibitor, as part of a Phase 1b study (NCT02795182). Both developed a newly identified syndrome of severe direct antiglobulin test (DAT) negative hemolysis and reticulocytopenia associated with transfusions, requiring cessation of the anti-PD-1 antibody and initiation of additional therapies.
Case descriptions
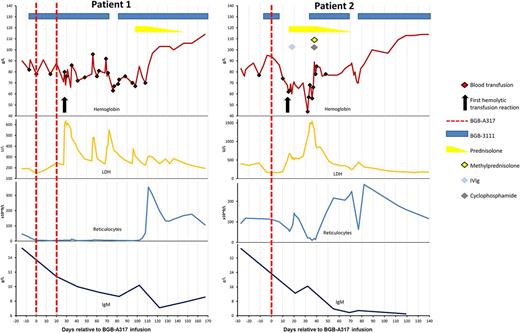
Patient 1 is a 62-year-old female with WM who had previously received rituximab, cyclophosphamide and dexamethasone, and later rituximab and bendamustine (BR). After her most recent relapse she was treated with BGB-3111 followed 7 days later by BGB-A317. Four weeks after administration of the anti-PD-1 agent she developed hemoglobinuria, flank pain and fever during a red cell transfusion, with biochemical markers of hemolysis. DAT was negative and no red cell antibodies were identified. BGB-A317 was ceased, however hemolysis persisted and she required 26 further red cell transfusions. She was profoundly reticulocytopenic throughout this time. Prednisolone at 1mg/kg was initiated with a rapid resolution of hemolysis. The steroids were weaned and ceased over 7 weeks and she remains 4 months transfusion independent. Her WM is responding to single agent BGB-3111.
Patient 2 is a 67-year-old female initially managed for WM with fludarabine, cyclophosphamide and rituximab. First relapse was treated with BR followed by autologous transplant. After a 3-year remission, a further relapse led to initiation of BGB-3111 then BGB-A317. Fourteen days after infusion of BGB-A317 she developed a hemolytic transfusion reaction with hemoglobinuria and elevation of biochemical markers of hemolysis. The blood film was unremarkable, DAT was negative and no antibodies could be identified. Reticulocytopenia was notable and worsened with progressing hemolysis. Ongoing hemolysis of transfused and native red cells was refractory to high dose prednisolone and intravenous immunoglobulin. Life-threatening anemia (nadir hemoglobin 4.4 g/dL, peak LDH 1544 U/L) prompted therapy with cyclophosphamide and pulse methylprednisone. BGB-3111, briefly withheld due to thrombocytopenia, was reinitiated. These measures were effective and the hemolytic anemia resolved over 3 weeks. Prednisone was weaned and the patient remains 4 months transfusion independent. Notably, a brief cessation of BGB-3111 at a later stage resulted in a transient recrudescence of hemolysis.
Discussion
Immune related adverse events are well described with immune checkpoint inhibitors, including a few reported cases of autoimmune hemolytic anemia. In our patients, there was a clear temporal relationship between the BGB-A317 and hemolysis, which started 28 and 14 days after the first dose. Subsequent resolution of the syndrome took 80 and 55 days from the final dose of BGB-A317, equivalent to 5 times the estimated elimination half-life of 11 to 17 days. The response to glucocorticoids and cyclophosphamide support an immune mechanism initiated by checkpoint inhibition. The profound reticulocytopenia and marked erythroid hypoplasia suggest that erythroid precursors may have been a predominant target of the immune attack.
No other cancer patients treated with BGB-A317 have developed a similar reaction to the two patients, who were the first worldwide with WM to receive the agent. These observations suggest a pathophysiological role of WM, which is known to be associated with immune dysregulation and autoimmune phenomena. We hypothesize that control of the underlying lymphoma by BGB-3111 contributed to resolution of the hemolysis.
Lymphoproliferative disorders such as WM involve complex aberrations of immune function and inhibition not limited to the malignant clone. The introduction of therapeutic agents harnessing the immune system in this patient population should be approached with caution, as demonstrated by the unexpected and severe adverse events suffered by these two patients.
Tam: Roche: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding. Huang: BeiGene: Employment. Lin: BeiGene: Employment. Hilger: BeiGene: Employment. Trotman: Roche: Membership on an entity's Board of Directors or advisory committees, Other: All positions non-remunerated, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Funding facilitating research paid to third parties. All positions non-remunerated; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Position non-remunerated; Janssen Cilag: Membership on an entity's Board of Directors or advisory committees, Other: Funding facilitating research paid to third parties. All positions non-remunerated, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal